Etching In Metallography
Electrolytic polishing is the best way to polish very soft materials which are prone to smearing and deformation. It can be easily applied to objects of complex shape. Materials that work well for electropolishing or etching include soft austenitic stainless steels, aluminum and aluminum alloys, copper and copper alloys, among others. Shorter preparing time is needed for electrolytic polishing and etching process compared to traditional mechanical preparation. The primary requirement for electropolishing is that the specimen be conductive.
Polished metal specimens usually show no structural characteristics. Metallographic etching of the metal surface is done to make visible the crystalline structure of the metal and to produce optical contrast between the various constituents.
Electropolishing is also commonly applied to the preparation of thin metal samples for transmission electron microscopy because electropolishing does not cause mechanical deformation of surface layers usually observed when mechanical polishing is used.
Etchants are composed of organic or inorganic acids, alkalis, or other complex substances, in a solvent such as water, alcohol, glycerine or glycol.
Over the years, many different reagents have been developed for specific purposes and materials. Therefore they must be chosen carefully to reveal the desired structure. The specimen is either held by tongs and immersed, with the polished face down into a small petri dish partly filled with the etching reagent, or swabbed with cotton wool, which has been saturated with the etching reagent. When etching, progress should be observed and timed, depending on the materials and reagent, this could vary from a few seconds to a minute or more.
After etching, the specimen should be thoroughly washed in water or alcohol and then dried using a hot air blower. The specimen should be carefully stored and cared for in a desiccator, to prevent oxidation and scratching.
Electrolytic Sample Preparation
Preparing Electrolyte
Each specimen may require different types of electrolytes. Proper electrolyte should be selected for specimen. Please see below table for common electrolytes. Fill electrolyte container of ELOPREP with correct electrolyte and place it on polishing unit. If you have different specimens which required different electrolytes, you can use additional electrolyte containers. Thus, you do not have to change electrolyte for different types of specimen, you only need to change electrolyte container.
Choosing Masks
Acid resistant masks are used for determining polishing area of specimen. There are specific aperture on the masks. Electrolyte will contact the specimen surface from this aperture thus only this area will be polished. Masks are available with 0.5-1-2 and 5 cm2 aperture size. Mask should be placed on cathode and specimen should be placed on the mask.

Adjusting Flowrate
Flowrate of electrolyte should be adjusted before operation. In other words, electrolyte should be reached upper level of the mask without any turbulance. Otherwise, polishing operation cannot be done properly. To adjust flowrate, press Pump button without placing sample. If the flow rate is not enough, increase the set value until the flow is sufficient.
Preparing Specimen
Surface of specimen should not be rough for electrolyic preparation. Specimen should be grinded before electrolytic preparation.
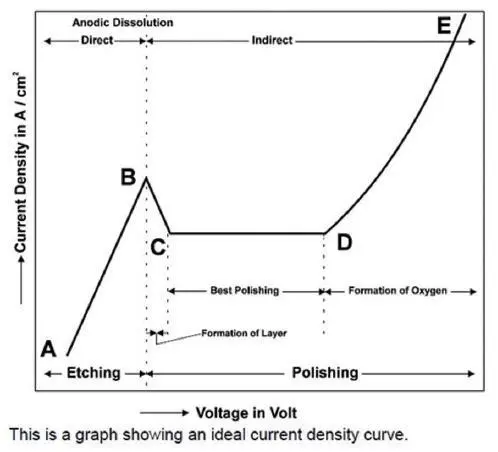
Determining Correct Voltage
Voltage is one of the most important parameter for electrolytic polishing. The ELOPREP has a scanning function for easy and exact determination of the parameters. If you do not know the polishing voltage to be set for your sample, you can use the scanning function to determine the exact voltage.
- Place specimen on the mask. Be sure that the aperture is covered and closed completely.
- Place the anode arm on the top of the sample to ensure good contact.
- Enter Scan menu, set scanning voltage and flowrate parameters and press Start.
- After a while, the ideal polishing voltage for your specimen will be seen on the screen.
Electrolytic Polishing & Metallographic Etching
ELOPREP has 4 different operation modes; only polishing, only etching, automatic etching after polishing and external etching.
- Place specimen on the mask. Be sure that the aperture is covered and closed completely.
- Place the anode arm on the top of the sample to ensure good contact.
- Enter Polishing&Etching menu. Set parameters: Voltage, flowrate, mask type and time.
For only polishing: Set only polishing time and polishing voltage. Do not set etching time and etching voltage, these should be zero “0”. Press start and operation will be completed automatically. The specimen must be washed after polishing operation is completed.
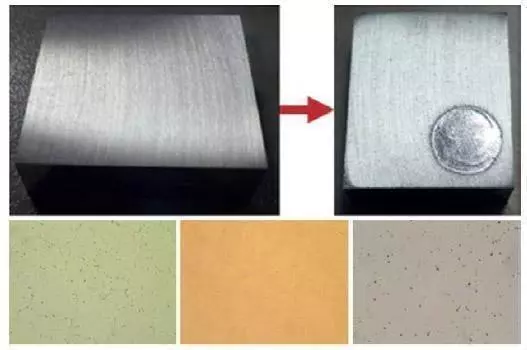
Polished surface and microstructure (Microstructure of Al-Cu-Steel)
For only etching: Set only polishing time and polishing voltage. Do not set etching time and etching voltage, these should be zero “0”. Press start and operation will be completed automatically. The specimen must be washed after etching operation is completed. Microstructure of etched surface can be seen as below:

Microstructure of etched surface (Microstructure of Al-Cu-Steel)
For automatic etching after polishing: Set polishing voltage, polishing time, etching voltage and etching time. Do not set etching time and etching voltage. Press start. Etching will be done automatically after polishing operation is completed. The specimen must be washed after etching operation is completed. For external etching: If polishing and etching electrolytes different than each other, in this case external etching unit can be used.
- Fill external etching unit with electrolyte.
- Connect cables of external etching unit to control unit.
- Enter External Etching menu. Set etching voltage and time.
- Hold the sample with scissors.
- Plunge the sample into the external etching unit. As soon as the sample contacts with the electrolyte the process will start automatically.
- As soon as the set etching time is reached an audible signal notifies the operator that the process is completed. Wash your specimen after etching is completed.

Metallographic Etching Reagents for Microscopic Examination of Metals
Note: Many etching reagents are powerful and must be handled with care.
| Class | Use | Formula | Volume | Cell Voltage | Time | Remark |
|---|---|---|---|---|---|---|
| Group I (Electrolytes Composed of Perchloric Acid and Alcohol With or Without Organic Additions) | ||||||
| I-1 | Al and Al alloys with less than 2 percent Si, Steels, carbon alloys, stainless steels | Ethanol (95 percent) | 800ml 140 ml 80 ml | 30 to 80 | 15 to 60s | Nickel cathode |
| I-2 | Stainless steel and aluminum | Ethanol (95 percent) Perchloric acid (60 percent) | 800ml 60 ml | 35 to 80 | 15 to 60s | |
| I-3 | Stainless steel | Ethanol (95 percent) Perchloric acid (65 percent) | 940ml 60 ml | 30 to 45 | 15 to 60s | |
| I-4 | Steel, cast iron,Al,Al alloys Ni, Sn, Ag, Be, Ti, Zr, U Heat-resisting alloys | Ethanol (95 percent) 2-butoxy ethanol Perchloric acid (30 percent) | 700 ml 100ml 200 ml | 30 to 60 | 15 to 60s | One of the best formulas for universal use |
| I-5 | Steels-stainless, alloy, high speed, Fe, Al, Zr, Pb | Ethanol (95 percent) Glycerin Perchloric acid (30 percent) | 700ml 100ml 200ml | 15 to 50 | 15 to 60s | Universal electrolyte comparable to I-4 |
| I-6 | Al, Al-Si alloys | Ethanol (95 percent) Diethyl ether Perchloric acid (30 percent) | 760ml 190ml 50ml | 15 to 60 | 15 to 60s | Particularly good with Al-Si alloys |
| Group II (Electrolytes Composed of Perchloric Acid and Acetic Acid in Varying Proportions) | ||||||
| II-1 | Cr, Ti, Zr, U, Fe, steel, carbon alloy, stainless steel | Acetic acid (glacial) Perchloric acid (60 percent) | 940 ml 60 ml | 20 to 60 | 60s | Good general- purpose electrolyte |
| Group III (Electrolytes composed of Phoephoric Acid in Water Organic Solvent) | ||||||
| III-2 | Pure cooper | Distilled water Phosphoric acid (85 percent) | 175ml 825ml | |||
| III-10 | Cu and Cu-based alloys | Distilled water Ethanol (95 percent) Phosphoric acid (85 percent) | 500ml 250ml 250ml | |||
| Group IV (Electrolytes Composed of Sulfuric Acid in Water or Organic Solvent) | ||||||
| IV-1 | Stainless steel | Water Sulfuric acid | 250ml 750ml | 1.5 to 6 | 1 to 2 min | |
| Iv-6 | Stainless steel, aluminum | Water Glycerin Sulfuric acid | 220ml 200ml 580ml | |||
Metallographic etchants for stainless steel
Here are some commonly used metallographic etchants for stainless steel:
- Nital: Nital is a commonly used etchant for stainless steel. It is a mixture of nitric acid and ethanol, typically with a concentration of 2-10% nitric acid. Nital is effective in revealing the general microstructure of stainless steel, including grain boundaries and different phases.
- Marble's Reagent: Marble's Reagent, also known as Iron Chloride, is another popular etchant for stainless steel. It consists of a mixture of ferric chloride and hydrochloric acid. Marble's Reagent is useful for revealing carbides and intermetallic phases in stainless steel.
- Vilella's Reagent: Vilella's Reagent is a mixture of 1 part hydrochloric acid, 1 part ethanol, and 1 part water. It is often used to reveal the grain boundaries in stainless steel.
- Aqua Regia: Aqua Regia is a mixture of nitric acid and hydrochloric acid. It is a highly aggressive etchant that can be used to reveal the grain structure and intergranular corrosion in stainless steel.
- Picral: Picral is a mixture of picric acid and ethanol. It is commonly used to etch stainless steel to reveal the delta ferrite phase, which is often present in austenitic stainless steels.
Metallographic etchant for copper
Here are some commonly used metallographic etchants for copper:
- Ferric Chloride: Ferric chloride is a popular etchant for copper. It is available in crystal or liquid form and can be dissolved in water to form a solution. Ferric chloride is effective in revealing the grain structure and boundaries in copper.
- Ammonium Persulfate: Ammonium persulfate is another commonly used etchant for copper. It is a white crystalline powder that can be dissolved in water to form an etching solution. Ammonium persulfate is suitable for revealing the grain boundaries and structure in copper.
- Nitric Acid: Nitric acid can be used as an etchant for copper, although it is not as commonly used as ferric chloride or ammonium persulfate. It is a highly corrosive and hazardous chemical, so proper safety precautions should be followed when handling it. Nitric acid is effective in revealing the microstructure and grain boundaries in copper.
- Cupric Chloride: Cupric chloride is a specific etchant used for revealing the microstructure and grain boundaries in copper. It is less commonly used than ferric chloride or ammonium persulfate but can provide good results.
Metallographic etchant for inconel 625
Here are some commonly used metallographic etchants for Inconel 625:
- Kalling's reagent: Kalling's reagent, also known as Kalling's No. 2 or K2 etchant, is a commonly used etchant for nickel-based alloys like Inconel 625. It consists of a mixture of 2% hydrochloric acid (HCl) and 98% ethanol. Kalling's reagent is effective in revealing the grain boundaries and other microstructural features of Inconel 625.
- Aqua Regia: Aqua Regia is a mixture of nitric acid (HNO3) and hydrochloric acid (HCl). It is a highly aggressive etchant and can be used to reveal the grain structure and other features in Inconel 625. However, caution should be exercised when using Aqua Regia due to its corrosive nature and safety hazards.
- Nital: Nital, a mixture of nitric acid (HNO3) and ethanol, is a versatile etchant that is also used for Inconel 625. A commonly used concentration is 2% nitric acid in ethanol. Nital can reveal the microstructure, grain boundaries, and other features of Inconel 625.
- Glyceregia: Glyceregia is a mixture of glycerol, hydrochloric acid (HCl), and nitric acid (HNO3). It is an effective etchant for revealing the grain boundaries and other features in Inconel 625. Glyceregia can be prepared by mixing 1 part glycerol, 1 part concentrated nitric acid, and 3 parts concentrated hydrochloric acid.
Metallographic etchant for titanium
Here are some commonly used metallographic etchants for titanium:
- Kroll's Reagent: Kroll's Reagent is a commonly used etchant for titanium. It consists of a mixture of 30 mL hydrofluoric acid (HF), 60 mL nitric acid (HNO3), and 10 mL water. Kroll's Reagent is effective in revealing the microstructure and grain boundaries of titanium.
- Weck's Reagent: Weck's Reagent, also known as Weck's Solution, is another widely used etchant for titanium. It is a mixture of 10 mL hydrofluoric acid (HF), 45 mL nitric acid (HNO3), and 45 mL water. Weck's Reagent is particularly useful for revealing the grain boundaries and alpha-beta phase morphology in titanium alloys.
- Vilella's Reagent: Vilella's Reagent is a mixture of 1 part hydrochloric acid (HCl), 1 part ethanol, and 1 part water. It is commonly used to etch titanium to reveal the grain boundaries and microstructure.
- Kroll's Solution: Kroll's Solution is a mixture of 10 mL hydrofluoric acid (HF), 10 mL nitric acid (HNO3), and 80 mL water. It is often used for revealing the microstructure and grain boundaries in titanium.
It's important to note that the selection of the appropriate etchant for your material depends on the specific application and the desired results. Different etchants may be more suitable for different material and/ or surface conditions. Proper safety precautions should be followed when handling and using etchants, as they can be hazardous.

